 Sixth installment summarizing Susskind's, Quantum Mechanics: The Theoretical Minimum.
Sixth installment summarizing Susskind's, Quantum Mechanics: The Theoretical Minimum.Chapter 1: Dirac was much smarter than I (introducing linear algebra).
Chapter 2: Quantum States (a.k.a., more linear algebra)
Chapter 3a: Linear Operators
Chapter 3b: Eigenvectors
Chapter 3c: Hermitians and Fundamental Theorem of QM
1. Again there is the sense that if I can just make it a little further, he'll connect this stream of math to something concrete so that all the rest will click. I feel like I'm reading 1 John.
Principle 1: Observable quantities in quantum mechanics are represented by linear operators. These have to be Hermitian as well.
Principle 2: The possible results of a measurement are the eigenvalues of the operator that relates to that observable. If a system is in the eigenstate ∣λ〉 , the result of a measurement has to be λ .
Principle 3: Distinguishable states are orthogonal vectors.
Principle 4: The probability of observing a value λ is 〈A∣λ〉2 That is the probability of observing a particular eigenvalue is the square of the overlap between the eigenvalue and that state in general.
2. So Susskind uses the spin operator as an example. A spin operator provides information about the spin component in a specific direction. There is a spin operator for each direction in which the measuring apparatus can be oriented.
So he asks what an appropriate "spin operator" might be for the "up-down" aspect of spin. For up, the value will be one for up and zero for down. For down, the value will be zero for up and -1 for down. This corresponds to the following matrix:
 |
| z matrix (up down) |
3. He derives the matrices for the "right left" and "in out" components as well. These three matrices constitute the "Pauli matrices."
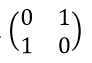 |
| x matrix (left-right) |
 |
| y matrix (in out) |




No comments:
Post a Comment